Why is Every Newborn Forced to Get the Dangerous Hepatitis B Vaccine?
The suppressed history behind the Hep B vaccine and the actual risks and benefits of it we are never told about.
Story at a Glance:
•The hepatitis B vaccine has been marred by controversy since its inception, particularly since it is now given to every newborn child despite minimal benefit being attributable to this policy.
•Remarkably, much of that controversy (e.g., Congressional hearings, mainstream news programs, and HIV vaccine contamination concerns) has been largely forgotten.
•The hepatitis B vaccine has long been associated with autoimmune disorders, particularly demyelinating ones. While the medical community has insisted for over 50 years that this link remains unproven and requires “further research”, evidence demonstrates this process indeed occurs.
•While the hepatitis B vaccine has reduced acute cases in high-risk demographics (e.g., intravenous drug users), there is no evidence it has done the same in newborns (as applicable circumstances are incredibly rare), and no evidence it has reduced chronic hepatitis cases (which is the justification for the entire program).
•As such, the official reason every newborn child gets an infant hepatitis B vaccine is not the actual reason why parents and doctors around the country were pressured to administer it to every newborn.
•Tomorrow, the blanket policy to give it to every newborn will at last be re-evaluated. To support this pivotal moment, this article will attempt to establish the actual risks and benefits of the hepatitis B vaccine, along with exposing the forgotten history behind the vaccine, which brought us to where we are now.
Note: an abridged version of this article can be read here.
In order to have a healthy and meaningful life, people need to have a unifying purpose behind everything they do. Recognizing the importance of this at a young age (as I saw many lacking one struggle greatly), I decided to devote myself to the pursuit of truth, regardless of where it took me. From this, I quickly realized how difficult this was, as on virtually every issue, there is a massive amount of ambiguity, which inevitably leads you reaching false conclusions produced from your existing biases.
Because of this, whenever I try to figure out why something “bad” is happening, I take numerous possibilities (often over a dozen) into consideration, and frequently never fully commit to any as I don’t feel a definitive case was made for any of them—an approach which lies in stark contrast to those who come across one explanation and immediately commit to espousing it (as it “makes sense”). Rather, I patiently wait and have faith I will eventually uncover the thread that ties all the disparate pieces together (which when finally revealed, is an immense source of joy).
Note: this is why, while I sometimes claim things are true, I am also quite deliberate in prefacing other statements with “I suspect” or “I believe.”
The hepatitis B vaccine for example, is one of the most controversial vaccines on the immunization schedule, as some of the strongest arguments both in favor and against vaccination exist for its current use. I’ve hence spent decades trying to figure out why we give it to every newborn in America, and have heard numerous compelling explanations to account for this, but never found one that appeared to explain everything.
Fortunately, two weeks ago, a reader finally provided the answer to this question—an answer I was obligated to publicize, as tomorrow on Thursday (9-18-2025), the ACIP (the independent advisory committee which decides which vaccines are “recommended” to America), after decades, will at last be seriously re-evaluating the appropriateness of giving it to all newborns.
A recent Highwire clip, in turn, highlights how controversial this subject is, as individuals on both sides have spoken out aggressively in favor of or in opposition to potentially changing the existing hepatitis B recommendation:
Note: at a contentious hearing today, the now dismissed CDC director (Daskalakis’s boss), when repeatedly pressed by Senator Rand Paul to do so, was unable to provide any rationale for why we give every newborn the hepatitis B vaccine, despite widely decrying any attempt to overturn it—again illustrating the shaky ground this policy rests upon.
Hepatitis B Safety Concerns
Since entering the market, the hepatitis B vaccine has been marred with safety concerns, particularly after it was given to every child in America. What follows is a brief summary of some of those concerns:
•As early as 1976, one researcher cautioned that since autoimmunity is involved in the pathogenesis of hepatitis B infections, it they might also be provoked by molecularly similar hepatitis B vaccines. Since that time, many other papers have shown the vaccine provokes a wide range of autoimmune disordes.1,2,3,4,5
•One researcher, Bohn Dunbar (a respected vaccine researcher who was a medical school professor), after her brother and research assistant both developed autoimmune and neurological injuries from the vaccine in 1994, devoted herself to exposing the frequent pattern of autoimmune complications from the vaccine (e.g., “Dr. Dunbar has also been in contact with numerous physicians and research scientists from several countries who have independently described thousands of identical severe reactions occurring in Caucasian recipients of the vaccine”). In turn, due to both her prestige and ability to navigate the academic publishing system, she brought significant attention to this subject (e.g., see this 1999 Washington Post article).
•A 1998 Article in Scientist highlighted growing concerns threatening to derail the hepatitis B vaccine program, such as more and more people claiming it caused serious autoimmune diseases (e.g., rheumatoid arthritis [RA], optic neuritis, and multiple sclerosis [MS]), that one doctor had collected over 600 cases of this happening, and that in July, attorneys representing 15,000 people sued France’s government for exaggerating the vaccine’s benefits and downplaying its risks.
•Shortly after, France suspended hepatitis B vaccinations in schools (to assess if it could cause demyelinating diseases), which the WHO, the ACIP, and France’s medical associations all strongly condemned due to it weakening public confidence in the vaccine.
•In January 1999 (one of the last times major news networks still aired programs critical of pharmaceutical interests—as Clinton has recently legalized pharmaceutical companies buying them out), ABC news hosted an almost entirely forgotten program on the hepatitis B vaccine, which featured a chief CDC and Merck official (who claimed mass vaccination justified preventing a few hepatitis cases and that no injuries attributed to the vaccine were actually caused by it) along with many vaccine injured patients, including both injured adults and parents of severely injured children.
Shortly after, at a May 1999 Congressional hearing discussing the merits of universal hepatitis B vaccination of newborns, in addition to many espousing the need for it, the following objections were raised in testimonials from experts and vaccine-injured parties who testified against the practice:
Severe Adverse Reactions: Numerous serious side effects were discussed, including infant death, seizures, autism, dysautonomia, MS, RA diabetes, and rare cases of liver cancer in children post-vaccination (along with established mechanisms for the autoimmune responses). VAERS data in turn, indicated over 8,000 reactions, including 43 deaths in children under 2 in 1997. In contrast, there were only 95 annual hepatitis B cases in this demographic (with comparable, or smaller, numbers seen in other datasets)—suggesting injuries vastly outweighed prevented hepatitis cases (particularly since less than 1% of injuries are typically reported to VAERS and infant deaths from hepatitis were virtually non-existent in the pre-vaccination era).
Note: at each point in time where the safety of the hepatitis B vaccine was questioned, the same pattern of injuries (e.g., characteristic autoimmune disorders and infant deaths) in VAERS was cited, with the total number of them continually increasing as the years went by.Inadequate Safety Monitoring and Research: Adverse reaction reports were often ignored or dismissed, with short trial durations (4–5 days), missing delayed reactions like MS or diabetes, which may appear years later. No studies focused on newborns or genetic predispositions, and underreporting was common due to physician denial.
Lack of Informed Consent and Coercion: Parents received inadequate risk information, with CDC materials omitting serious adverse effects listed in manufacturer inserts. Newborns were vaccinated without parental consent, and some faced coercion, including threats of social services intervention.
Questionable Mandate: Vaccinating low-risk newborns for an adult-associated disease is inappropriate, particularly since immunity can wane before adolescence and 10–30% of individuals fail to produce antibodies, questioning efficacy.
Conflicts of Interest: Pharmaceutical influence on health agencies raised doubts about study objectivity.
All long-term research into the safety of the vaccine was being stonewalled (e.g., see this testimony), yet the medical community argued the lack of robust longterm safety studies actually proved the vaccines were “safe” but promised to do future research to determine if the vaccines were safe (which 25 years later still has not happened).
Vaccine Injury Compensation Issues: The National Vaccine Injury Compensation Program denied most claims, leaving victims unsupported despite a $1 billion trust fund, with restrictions limiting filings for hepatitis B vaccine injuries (e.g., see this testimony).
Note: most of the above has also been said about many other vaccines over the decades. Likewise, in a 1999 testimony before the Ohio legislature, another physician noted that most of the deaths following hepatitis B were classified as SIDS (a condition extensive evidence shows is strongly linked to vaccination) yet SIDS was almost always defined as occurring between 1 month to 1 year of age, and that prior to the hepatitis B vaccine being given to newborns, it rarely if ever affected children under 2 months of age—however in VAERS, many cases labeled as SIDS were reported in infants under one month of age following the vaccination. Furthermore, a relatively unknown 2004 study found the hepatitis B vaccine increased the risk of at 7-12 months of age by 1.81X (although this may in part have been due to vaccine induced immune suppression making African children more vulnerable to existing infections).
Many of the testimonials were quite riveting:
•This woman’s son developed seizures, then neurologic disorders, then autism after the vaccine.
•This nurse developed chronic inflammatory demyelinating polyneuropathy (losing the ability to walk) along with multiple types of autoimmune disorders.
•This adolescent girl became disabled from the neurological and autoimmune complications of the vaccine
This father’s daughter died immediately after vaccination:
Furthermore, one of the Congressional witnesses, produced an excellent (referenced) summary of the major issues with the hepatitis B vaccine which included two cases he’d observed it causing encephalomyelitis (resulting in a two week coma for one, a four week coma for the other, along with optic neuritis and significant neurological disability for both, and no clear conventional explanation for what had occurred) along with many cases of it causing chronic fatigue syndrome. He then compiled a list of dozens of studies demonstrating that the hepatitis B vaccine was linked to a myriad of autoimmune disorders, as did two other author decades later (e.g., in a 2015 textbook on the subject or within a natural health database). Those studies (which are likely only the tip of the iceberg) are as follows:
Multiple Sclerosis,1,2,3,4,5,6,7,8,9,10,11,12,13,14 myelitis,1,2,3,4,5,6,7,8,9,10,11,12,13,14 encephalitis,1 encephalomyelitis,1 optic neuritis,1,2,3,4,5 Guillain–Barré syndrome,1,2,3,4,5,6,7,8,9 neuropathy,1,2,3,4,5,6,7,8,9,10 myopathy,1,2,3,4,5,6,7,8 Myasthenia Gravis,1,2,3 APMPPE (an eye disease)1 uveitis1,
Arthritis,1,2,3,4,5,6,7,8,9,10,11,12,13 Lupus,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 juvenile dermatomyositis,1,2,3,4,5 macrophagic myofasciitis,1 polyarthralgia-myalgia,1 Still’s disease1
Vasculitis (general,1,2,3,4 pulmonary and cutaneous,1,2 Churg-Strauss,1,2 Henoch–Schonlein purpura,1 Kawasaki’s disease1 polyarteritis nodosa1), hemolytic anemia,1 thrombocytopenia,1,2,3,4,5,6,7 antiphospholipid syndrome1,2
Lichen planus,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 lichen striatus,1 bullous pemphigoid,1,2 erythema multiforme,1,2,3 erythema nodosum1 Gianotti–Crosti syndrome,1,2 alopecia,1,2 buchal aphthosis1
Chronic Fatigue Syndrome,1,2,3,4,5 Fibromyalgia,1 Graves’ disease,1,2 Sjogren's syndrome1
Hepatitis,1,2,3,4 glomerulonephritis,1 pancreatitis1 pneumonitits1
Note: the hepatitis B vaccine has also been linked to a variety of other disorders not classically classified as autoimmune disorders such as seizures,1,2 Bell’s palsy,1,2 cerebellar ataxia,1 tic disorders,1 anorexia,1 tufted angioma1 and to increase common childhood illnesses (e.g., one study found a 1.6X increase in acute ear infections and a 1.41X increase in pharyngitis and nasopharyngitis).
As mentioned above, since the start, it was widely believed that the autoimmune conditions the hepatitis B vaccine caused were due to its antigen having a significant overlap with human myelin (particularly since many of the autoimmune disorders associated with the vaccine were also observed to sometimes occur from a hepatitis B infection).
The molecular mimicry of the vaccine, in turn, was a hotly debated topic that all medical authorities denied was occurring. As it was not possible to assess with the technology of the time, the absence of evidence for it was treated as evidence that it did not.
Note: a definitive 1994 report by the Institute of Medicine noted that while preliminary data existed for many of the injuries attributed to the hepatitis B vaccine, no further research had ever been done, so there was insufficient evidence to prove or disprove a link between these conditions, and concluded its report on the safety of the childhood vaccines by declaring that the lack of adequate data regarding many of the adverse events under study was of significant concern to the committee (but as you might guess, the studies they requested still have not been done).
However, while it was not possible to assess then, a 2005 study showed the hepatitis B vaccine did, indeed, have a significant overlap with myelin and more importantly, that 60% of its recipients also developed immune reactivity to the myelin coasting their nerves (which in the majority of cases persisted for over 6 months). Sadly, by the time this was discovered, the use of the hepatitis B vaccine had been normalized, and public debate on its safety or autoimmunity risk had long since ended.
Note: a 2001 review also highlighted the potential for this mimicry to cause MS, while another paper highlighted that the vaccine overlaps with Epstein-Barr Virus in a manner which could provoke demyelinating disorders in recipients with existing EBV infections.
Likewise:
•A 2005 VAERS study comparing adults who’d received a tetanus-containing vaccine to a hepatitis B vaccine found they were much more likely to develop a variety of autoimmune disorders (5.2X for MS, 18X for RA, 14X for optic neuritis, 9.1X for lupus, 7.2X for alopecia, 2.6X for vasculitis, and 2.3X for thrombocytopenia). A similar 2002 study found a 6.1X increase for chronic arthritis (persisting for at least one year), which affected women 3.5X as much as men, and on average occurred 16 days after vaccination.
•A 2002 study found individuals who received a hepatitis B vaccine, within the next two months, were 1.8 times as likely to experience a demyelinating event.
•A 2015 study found cases of MS in France rose by 65% in the years following an aggressive national campaign to increase hepatitis B vaccination rates, and that a statistically significant correlation existed between the number of hepatitis B vaccine doses given and the number of MS cases 1-2 years later.
•A 2004 study analyzed primary care records from across England to compare 163 MS patients with 1,604 randomly selected matched controls without MS. It found that MS patients were three times more likely to have received the hepatitis B vaccine within three years of symptom onset, with no similar risk linked to tetanus or influenza vaccines—indicating this was a specific issue with the hepatitis B vaccine.
•A 2009 study in children found that the GSK’s hepatitis B vaccine, which contains five times more yeast protein antigen than other brands, was associated with a 2.77X increased risk of developing MS in vaccine-compliant children. A smaller increase (1.5X) was observed for other CNS inflammatory demyelinating disorders in children who adhered to recommended vaccination schedules.
Additionally, the hepatitis B vaccine has also been repeatedly linked to autism and other developmental disabilities:
• In a June interview with Tucker Carlson, Secretary Kennedy revealed that in 1999, the CDC conducted a study which found that receiving a hepatitis B vaccine in the first 30 days of life caused a 12.35X increase in autism. As this was unacceptable, they conducted numerous attempts to adjust the data to hide the risk, but were unable to make the link go away, gave up, and never published it.
An abstract of a 1999 study (which is likely what RFK was referring to) was subsequently made available to a Florida Congressmen who had worked with vaccine whistleblowers, which showed (via the CDC’s private VSD database) that when infants received the highest doses of mercury containing vaccines (compared to those who had not been vaccinated), there was a 1.8X increase in neurologic development disorders, a 7.6X increase in autism, a 5.0X increase in nonorganic sleep disorders and a 2.1X increase in nonorganic sleep disorders.
•A 2007 study of 1824 children found boys who received the hepatitis B vaccine (prior to 2000 when it still used thimerosal) were 9 times as likely to have a developmental disability.
•A follow-up 2010 study found neonatal hepatitis B vaccination (compared to no hepatitis B vaccination or simply getting it later in life) made children 3 times as likely to develop autism. Finally, a 2017 study found mercury containing hepatitis B vaccines at the start of life increased the risk of autism by 4.6-6.7X (along with a 2015 study which found they increased the risk of developmental delays by 1.6X-1.7X along with a 2016 followup which estimated this equated to over a trillion in healthcare costs).
Similar results were also seen in animals:
•A 2010 monkey study determined that the vaccine caused a significant delay in the acquisition of root, snout, and suck reflexes (critical processes for development).
•A 2016 mouse study found the vaccine impaired neurogenesis, behavioral performances and hippocampal long-term potentiation which simultaneously increasing brain inflammation (which was proportional to the neurologic damage which occurred). A follow-up 2018 study determined many of these effects (particularly in the hippocampus) were a result of elevated IL-4.
Note: a 2013 study found that hepatitis B vaccination spiked their inflammatory CRP levels, and in 22 out of 70 infants, this increase was large enough to pass the diagnostic threshold for sepsis.
In contrast, the licensing studies for the vaccines only monitored for side effects during a short window long before these side effects would emerge (typically 4-5 days), did not use actual placebos (e.g., the original trials used either aluminum or aluminum and albumin1,2,3 while the later ones compared the vaccine to other “safe” vaccines).
The package insert of Merck’s vaccine noted that in the first 5 days, 17% of adults reported injection site reactions (e.g., pain, soreness, bruising, nodule formation), while 15% of adults and 10.4% of children reported systemic adverse reactions (e.g., fatigue/weakness, headache, fevers above 100°F, malaise, nausea, diarrhea, pharyngitis, upper respiratory infection).
The package of GSK’s vaccine noted after 4 days, 22% of recipients had injection site soreness, 14% had fatigue, and between 1-10% reported dizziness, headaches, and either redness, induration, or swelling at the injection site. Additionally, a variety of more severe conditions were reported in <1% of injections (e.g., anorexia, somnolence, hypotension, a wide range of gastrointestinal conditions, hives, irritability, and weakness). Finally, in adults with diabetes, 3.8% had serious systemic side effects (compared to 1.6% of controls).
In a 2018 trial of infants receiving the GSK hepatitis vaccine at birth, the following symptoms were reported within 48 hours: pain (9%), erythema (20%), swelling (4%), irritability (20%), vomiting (23%), diarrhea (12%), feeding difficulties (17%), drowsiness (28%), restlessness (31%), and fever ≥38°C (0.7%).1,2
Note: the 2018 trial, and a 2002 trial (where 32% of the infants developed drowsiness post vaccination) were recently cited by the CDC to demonstrate vaccine safety.1,2 However, as the now independent ACIP members pointed out, in practice, these seeming innocuous effects are often immense impactful (e.g., unexplained fevers can trigger an invasive sepsis workup which terrifies the parents, feeding difficulties are immensely challenging for the mother and often the infants long term health, and drowsiness or piercing cries can indicate serious neurological issues)—and per the CDC’s data, severe forms of these all occurred in at least 1% of infants.
Finally the only manufacturer trial I know of which evaluated the long term effects of hepatitis vaccination, was a fairly recent trial for a hepatitis vaccine (approved in 2017) which found after 7 months, 6.2% of its recipients had a serious adverse event (compared to 5.8% of those receiving GSK’s vaccine), which while quite concerning, were “insignificant” since they were comparable to a “safe” vaccine.1,2,3
In short, despite the fact that most of the research that should have been done never was, it is fair to say from what has been done that the risk profile of this vaccine suggests significant benefit must be seen from it to justify it being given to every newborn infant.
Determining The Risks and Benefits of Vaccines
With standard medications, calculating their risk-benefit ratio requires comparing the likelihood of treating an existing illness with the severity of the untreated disease to the probability of a course of the medication injuring the recipient. In many cases, this is a surprisingly difficult calculation to make, particularly since, on both ends, you have to account for the relative frequency of minor, moderate, severe, and lethal injuries and those that occur immediately, in the near future, and years down the road.
As such, while in many cases, the risks and benefits are fairly clear cut, with many other medications, we simply don’t know (or it takes decades to figure them out)—particularly since the pharmaceutical industry will always find the most favorable and deceptive way to portray the risks and benefits of their products positively.
With vaccinations, this calculation becomes much harder to do as the benefit is anything but certain (as you may never catch the illness and vaccines often fail to prevent their target illness), while conversely, since vaccines function by permanently altering the immune system, many of the side effects from doing so emerge long after clinical trials for vaccines evaluated vaccinated and unvaccinated cohorts.
Note: the above chart is still a gross oversimplification because it needs to be done for each cohort (e.g., different patient demographics have higher risks of an infectious disease, complications from the disease, and likewise, many have different responses to the vaccine). Likewise, this calculation needs to be done for each severity of a disease reaction (the mild, moderate, severe, or lethal) how long they persist (e.g., are they short term, medium term or permanent) and how hard they are to treat and then find some type of way to accurately compare them to the risks of the each vaccine (which unlike the potential risk of a disease, are a guaranteed exposure since everyone gets the vaccine regardless of disease status), along with taking into account vaccines that become more likely to injure (e.g., by causing autoimmunity) with successive injections and that many individuals have pre-existing vulnerabilities to vaccines (or an inability to develop immunity from them). Additionally, in many cases, the potential benefit of a vaccine is so small that the economic cost of giving millions of units of it out alone outweighs any potential benefit it can offer, it’s arguably not even necessary to proceed to the complex risk-benefit calculation (which rarely, if ever, will take into account the financial costs of the injuries).
Because of this, it is extremely difficult to calculate the risks and benefits of vaccines accurately, and instead we are either given extremely selective pictures of the risk/benefit profile (which focus on the aspects of it which drive vaccine sales) or simply robotically told “safe and effective” without that phrase ever being defined—all of which again illustrates how often a complex subject will be distilled into a simple soundbite that excludes most of the pertinent points.
In the case of the hepatitis B vaccine, on the surface, of the vaccines on the market some of the strongest arguments exist for it as:
•Large numbers of people get hepatitis B each year.
•Chronic hepatitis B is a disease that can severely impact one’s quality of life, and there is no good cure for it.
•The vaccine prevents hepatitis B.
Note: there is only one other vaccine on the CDC schedule to which all of the above points apply (although the severity of the illness it prevents is not comparable to chronic hepatitis B).
In turn, once the hepatitis B vaccine came out, hepatitis B rates dropped across the country, and the CDC (and the public health profession) has long considered this vaccine to be one of their crowning achievements and hence something they will fight to the death to protect.
Unfortunately, when you look under the microscope, many aspects of the above narrative fall apart, particularly when it is given to newborn children—which is why the vaccine is so controversial.
Note: because all vaccines are “safe and effective,” the public rarely learns that the risk/benefit ratio of vaccines varies widely, with some being arguable justifiable, while others are beyond a doubt unconscionable to mass distribute to our children. For that reason, I provided a comparison of each recommended vaccine’s risks and benefits here.
Hepatitis B Distribution
Note: the hepatitis B virus produces a few key proteins that are either produced by infection or vaccination. In almost all hepatitis B studies, the presence of absence of those antigens and antibodies to them (e.g., the surface protein hepatitis B uses to infect cells) is assumed to determine if someone previously had a hepatitis B infection, was vaccinated, is immune or has a chronic hepatitis B infection, and if it is a high-viral load infection (which is often more severe). In this article, I will treat those assumptions as correct. Likewise, I’ve tried to present mainstream data here (which is likely biased in favor of the vaccine) as it is more than sufficient to illustrate the serious issues with the vaccine.
Transmission of hepatitis B requires blood-to-blood contact, and typically occurs through one of the following means:
•Unprotected sex—with this being a significant root of transmission in low to moderate prevalence areas (meaning not as many people have hepatitis B), the highest risk typically being attributed to anal sex (due to blood from a torn rectum entering the urethra)—although I have not found any studies which directly compared vaginal and anal transmission.
•Accidental blood exchanges (e.g., sharing drug needles, use of contaminated dental or medical equipment, healthcare workers accidentally pricking themselves with a contaminated needle, or contaminated blood transfusions). Of these, sharing needles is the most common, needle stick injuries carry a real risk to unvaccinated healthcare workers, while the other two are no longer an issue in the United States.
•Mother to child (vertical) transmission during childbirth, as there is a large exchange of blood during this process (whereas in the uterus, typically hepatitis B does not cross the placenta and infect the infant—something which only happens around 4-16% of the time1,2,3,4,5,6). This is the primary mode of transmission in high prevalence areas.
Note: hepatitis B can also be transmitted through contact with other infected fluids, sharing non-sterile sharp objects (e.g., razors, or tattoo and piercing equipment), or in high prevalence areas, close contact with infected children (due to skin breaks and mucosal contact). However, these are much rarer routes of transmission.
The Western world (e.g., Australia, America, Western Europe) in turn, is considered to be a “low prevalence” area (with rates of hepatitis B typically below 2%). In contrast, other areas (e.g., sub-Saharan Africa, East Asia, Southeast Asia, the Western Pacific, and certain Pacific Islands) are high prevalence areas, with rates sometimes exceeding 15%. In short, as transmission of hepatitis B varies greatly depending upon how common it is, its low rate in the United States potentially undermines the justification for mass vaccination against it.
However, while it is generally low in the United States, the higher prevalence is frequently seen in a few communities specifically:
•Immigrants from areas with high levels of hepatitis B.
•Men who engage in unprotected sex with numerous males. For example, a 1982 study of five STD clinics found 6.1% of gay men had active hepatitis B, and 52.4% had been previously exposed to it, the placebo arm of the hepatitis B vaccine trial found 28% of them without it then developed hepatitis B in the next 18 months—of whom a small fraction (6.1%) then developed chronic hepatitis B, and a physician in the 1970s reported over 10% of gay men who visited his clinic had hepatitis B.
Note: recent data on this is much harder to find, with the only study I found (from an American HIV vaccine trial) determining 0.11% had chronic hepatitis B infections, and over the next 1-2 years, 0.22% had a previously eliminated hepatitis B infection reactivate.
•IV Drug users (with studies finding chronic hepatitis B at rates between 3.5-20% in these communities, and one 1989 sampling of Baltimore, finding 80% of users had evidence of a past hepatitis B exposure).
•Inner city youth and young adults who engaged in risky behaviors (e.g., unprotected sex, prostitution, and shared drug needles). This for context was a huge problem in the 1980s (e.g., a 1989 sampling of San Francisco residents aged 20-44 revealed 14% had previous hepatitis B exposure), although most of the literature I found highlighted the issue was constrained to certain subsections frequently found in inner city areas (e.g., injection drug users, sex workers, and the homeless) rather than the entire population, and likewise was more common in certain races than others (e.g., whites had the lowest hepatitis B rates).
Note: a 2005 sampling of a high drug use neighborhood in New York City found 8% had evidence of a prior hepatitis B infection (6% overall, 12.1% amongst drug users), while 19.6% had evidence of past vaccination (22.6% overall, 13.4% of the targeted sample).
Hepatitis B Vaccine Efficacy
Given the distribution of hepatitis, there are essentially four strategies which can be pursued with it: giving it to everyone at childbirth, giving it to children as part of routine immunizations, giving it to adolescents or young adults, and giving it to high risk groups (e.g., individuals sharing drug needles), each of which is more likely than the previous to reduce hepatitis B in “high risk” groups.
Let’s start by looking at the math behind a well publicized argument between two physician Senators:
In this context, vaccination is justified under the logic that if a mother was not tested for hepatitis B prior to delivery and she has it, receiving the vaccine within 48 hours of birth reduces their risk of catching hepatitis B and developing a chronic case, which gives way to chronic health issues. However:
•Hepatitis B screening is routine for infants, mandated by many states, and routinely done at hospitals if the status is not known—making it impossible to know how many infants actually are untested (everyone I’ve polled believes it is well under 1%—but here I’ll assume it’s between 0.1-5%). Likewise, it’s quite rare for a mother to suddenly become hepatitis B positive late in her pregnancy.
•Second, the percentage of American mothers with hepatitis B is not known (and greatly varies by community), but one authoritative source says 0.09%, while the CDC says 0.2-0.5%
•With hepatitis B, you can either be HepEAg+ (more severe) or HepEAg- (less severe). Around 10% of mothers in the developed world are HepEAg+ (compared to around 25% in poorer nations).
•Per the CDC references, if HepEAg+, there is a 70%-90% chance of acquiring perinatal HBV infection, and a 85%-90% of becoming a chronic carrier. If HepEAg-, data is less clear, but normally around a 10% chance of transmission is cited, with some then becoming chronic.
Note: in older patients, due to their being able to mount a more robust immune response, the chance of an acute infection becoming chronic is much lower (e.g., the CDC claims 30% for children under <5 years and <1%–12% for adults).
•When a mother is infected with hepatitis B, she can transmit the virus to her child in utero (which happens 5-30% of the time—particularly if HepEAg+—although the exact figures are not known1,2,3,4). However, normally, it occurs during childbirth when more maternal blood mixes with the infant, at which point it is though a vaccine can bolster immunity to the new hepatitis infection fast enough for the infant to eliminate it.
•The HepB vaccine usually is thought to reduce the risk of mother-to-child transmission by around 75% (e.g., these publications show 68%, 72% and 82-86%). Generally, the vaccine is more effective in HepEAg- mothers (e.g., 10% more), but all the comparisons I’ve found also gave immunoglobulins.
Note: since the hepatitis B vaccine is not 100% effective, it is typically given with costly immunoglobulins in children of parents with hepatitis B—but as this rationale for mass vaccination is to catch children who were missed by screening, it is not applicable to the scenario Cassidy is advocating for.
•Of those with chronic HBV, about 15-25% may progress to develop severe illnesses like liver cancer or failure later in life as adults (e.g., one study found 9.26% of infants born to a HepEAg+ mother who were vaccinated and given immunoglobulin ultimately developed chronic hepatitis).
If you in turn, take the middle value of each of those figures (and trust they are not inflated), this works out to needing to vaccinate roughly a million children to prevent one case of maternal-to-fetal hepatitis B transmission, and over 6.5 million to prevent a severe outcome from a chronic infection. Conversely, as I showed above, severe reactions (not to mention all the minor ones) are far more common following vaccination.
Note: for decades, parents have requested to be exempt from giving this vaccine to their children if they test negative at the hospital for hepatitis B, yet, in many cases, the mandate is still upheld—suggesting undetected hepatitis B, despite presented as such, is not actually the primary motivation for vaccination.
The second benefit commonly cited for hepatitis B vaccination is that it can prevent children from hepatitis B exposures, either from repeated contact with infected family members, or accidental exposures (e.g., touching contaminated needles on the playground). While each of these is quite rare, I am unsure exactly how to calculate the odds of it. For example, while I was able to find one case where this happened to a 4-year-old boy in Spain, conversely:
•A 19 year study of 274 Canadian children, many of whom were unvaccinated and did not receive post-exposure prophylaxis, found none of them developed hepatitis B, C, or HIV from abandoned needles they picked up and pricked themselves with—including ones from individuals known to be infected.
•A review of 16 studies involving 1,565 community-acquired needlestick exposures reported only one case of HBV transmission—where the child did not receive post-exposure prophylaxis, and alternative routes of infection could not be excluded.
The third benefit cited is that newborn vaccination can prevent infection later in life (when the children are expected to be old enough to be exposed to IV drugs or unsafe sexual activity). Unfortunately:
•Not all vaccinated children develop antibodies to hepatitis B following vaccination (seroconvert), with existing references showing between 90-98% do, with the figures being the lowest for adolescents (e.g., in a 2012 study of urban youth, between 5.4-12.8% of urban youths did not form an antibody response to the hepatitis B vaccine). Furthermore, premature infants have much lower responses to vaccination (e.g., figures between 11%-84% have been reported depending on how small they are1,2,3,4).
Note: numerous studies also show that smaller infants are also much more vulnerable to vaccine injuries (both chronic illnesses and sudden death)—again calling into question the wisdom of vaccinating newborns.
•In many cases, these antibodies wane long before potential HepB exposures. For example, one study found roughly 50% lost antibody immunity by age 5, another found roughly 22.9% lost it after approximately 5 years, another found 59% lost it by 9 years of age and 76% lost it by 13 years of age, another found 56% lost it by age 20 and another found 49% lost it over 30 years. Furthermore, in premature infants, the loss is faster (e.g., 84.1% lost it in less than 3 years, 73.5% after 4-7 years, 27.7% after 8-11 years and 20% after 12 years), while conversely, in adults it’s slower (e.g., in people vaccinated with 3 or 4 doses as adults, 20‑30 years later only 9.9% had lost antibody immunity).
•Seroconversion does not guarantee immunity, but it is generally assumed to equate to a 90-100% protection. However, one astute paper noted that data across the world showed after infant vaccination, 1.5%-3.5% of those 3-15 years of age had developed a hepatitis B infection (they often recovered from), with higher figures being seen after the 15 year mark (e.g., 4.9% in Korea, up to 5.5% in Hong Kong University students, 8% in Taiwan, 11-19% in Aboriginal communities and depending on age). Likewise, in Pakistan, the rates were 13.39% after 11-20 years and 34.93% after 21-30 years.
All of this data in turn, reminds me of one of the earliest lessons from vaccinology. The original smallpox vaccine was given by scraping it into the arm, and before long, the medical field realized that if there was not a large skin eruption at the application site, the vaccine “did not take” and hence would not work (necessitating repeat smallpox vaccination until it did). Eventually, natural medicine practitioners noticed the severe complications of the vaccine occurred in those who did not have that skin reaction (sometimes quickly, sometimes long after), leading to them formulating some of the foundational laws of natural healing and propose that rather than the smallpox vaccine preventing smallpox, was simply a proxy for an intact immune system, so those in which the smallpox vaccine “took” would have been able to fight off a smallpox infection regardless.
Note: somewhat similarly, one of the most important studies on COVID vaccine injuries showed that teenagers who developed myocarditis from the vaccine, unlike their peers, were unable to develop neutralizing antibodies to the vaccine spike protein (allowing it to wreck havoc throughout their body)—which almost certainly would have made them also be much more vulnerable to a COVID infection.
Given that around 5% of hepatitis B cases become chronic (rather than the body fighting it off), a figure is in line with the percent of people who the vaccine “doesn’t work for,” this has made me wonder if a similar issue is occurring here with the response to the vaccine simply being a proxy for a functioning immune system rather than independently protective (particularly since the same factors which predispose someone to being a poor vaccine responder, such as immune suppression, specific genes and being young, also increase the likelihood acute hepatitis B will become chronic).
Most importantly, while mass hepatitis B vaccination has significantly dropped acute hepatitis B cases, between 1976 to 2016, as the CDC admits, the prevalence of chronic hepatitis (0.003%) has not changed in the United States.1,2 Additionally, the decline in hepatitis B may not be solely due to the vaccine as it’s decline mirrored the other blood born hepatitis despite no vaccine being made for hepatitis C1,2 (suggesting the infant vaccination campaign took credit for the other policies introduced at the time to mitigate the spread of blood-born infections).

The final (and most substantial) justification is to give it to adults at high-risk of exposure (e.g., when I entered medicine, my mentors, who were greatly opposed to vaccination, said the one vaccine I needed to get was hepatitis B to be protected from future needle stick exposure). In turn, numerous datasets demonstrate that adult vaccination significantly reduces hepatitis B rates in the at-risk adult population. For example:
•Studies from the 1980s and 1990s showed that up to 28% of health-care personnel (HCP) had serologic evidence of past or current HBV infection (who typically could not recall the source of the exposure), while after decades of policies to ensure they were fully vaccinated for hepatitis B, annual cases dropped from 10,000 in 1982 to 304 in 2004.
•Many of the clinical trials for the hepatitis B vaccines showed they significantly reduced the instances of hepatitis B in male homosexuals (e.g., a 1980 trial of 1,083 men found 1.4% of vaccinated developed hepatitis B over the next 18 months, whereas 18.1% of the placebo group did, a 1982 trial found that after 24 months, 3.2% of vaccinated did, while 25.6% of the placebo group did). Likewise, later community studies found the same (e.g., in 1993, 359 San Francisco men were tested for Hepatitis B, and of those who’d previously been vaccinated, only 2.7% had it).
•A systematic review found that hepatitis B vaccination reduced acute hepatitis B cases from 25% to 15.7% in intravenous drug users, and created a 31.89% reduction in past or resolved hepatitis B infections—highlighting that not all people in this population fully respond to hepatitis B vaccination. Likewise, in Scotland, giving hepatitis B vaccines to all prisoners played a key role in increasing vaccination rates amongst intravenous drug users (going from 16% to 77%), which correlated with a significant reduction in their hepatitis B rates and a 40% reduction in their individual risk of hepatitis.
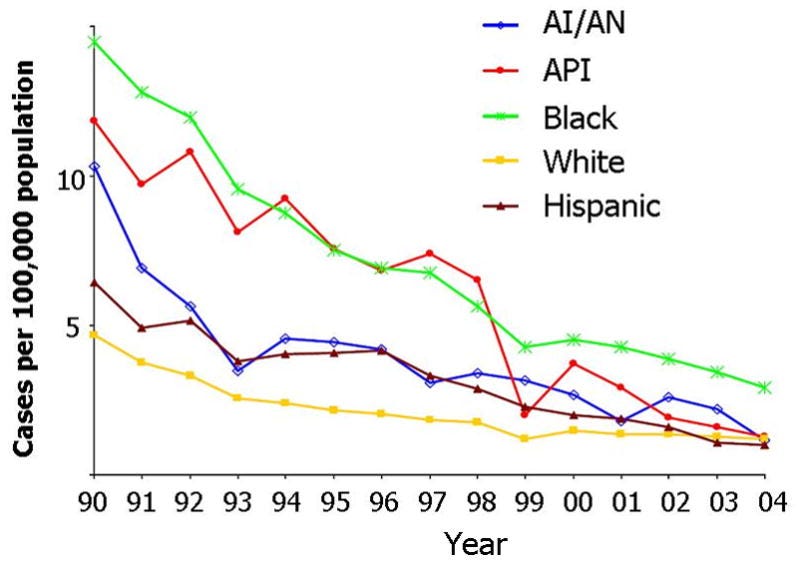
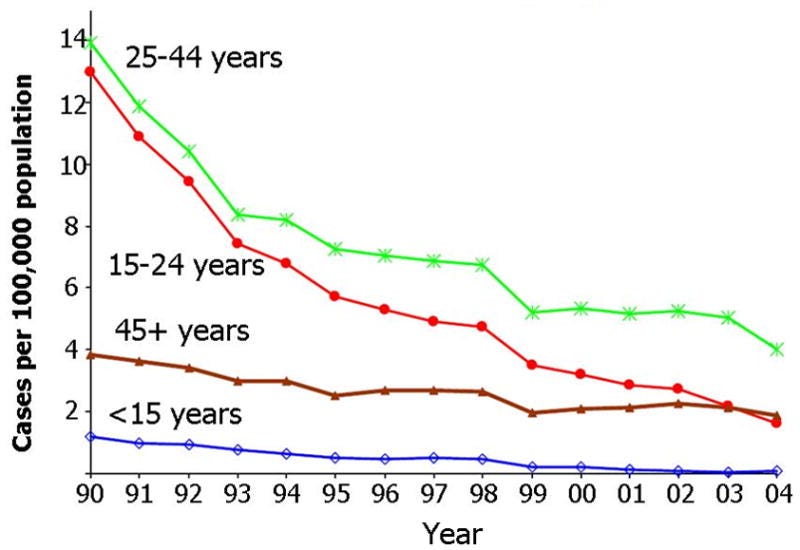
•From 1982 to 1998, in 4 USA counties, the reported incidence of acute hepatitis B declined by 76.1% from 13.8 cases per 100,000 in 1987 to 3.3 cases per 100,000 in 1998. Cases associated with injection drug use (IDU) decreased by 90.6%, in men who have sex with men (MSM) by 63.5%, and with heterosexual activity by 50.7%.
•Similar trends in new hepatitis B cases were also seen in other at-risk groups:
Note: critically however, the decline in new hepatitis cases was not seen in chronic hepatitis B cases (which has essentially remained unchanged from 19761,2 ).
All of this highlights a puzzling question. Why is it that the group (children) which have had the smallest benefit from this campaign has been the one most aggressively targeted by it? To understand this, we need to look at the entire history of the vaccine.
Hepatitis B Vaccine Design
Vaccines require producing large amounts of a target antigen, which in the case of hepatitis B, was challenging as it could only grow in humans and chimpanzees and could only be collected from the blood. In turn, exactly what transpired during the early days of the hepatitis B vaccine is a matter of significant debate. According to most sources, the vaccine was sourced from individuals with chronic hepatitis B infections and occasionally tested in chimpanzees. Conversely however, many other sources indicate there were direct blood exchanges between humans and chimpanzees either to develop the vaccine, to produce it initially, or to produce it at a lower cost for low-income markets.
This forgotten history (and the evidence supporting it), in turn, is detailed in this Twitter thread, these four Substack articles,1,2,3,4 and this brief documentary:
Note: the details in the above story are quite disturbing, if true, implicate many top public health officials (to the point it is plausible they would act in lockstep for decades to protect themselves) and may also apply to the early blood clotting products provided to hemophiliacs (which were established to be contaminated with HIV).
This was highly consequential as HIV is considered to have come from a related chimpanzee virus, and both the emergence of AIDS matched the original hepatitis B vaccine trials.1,2
Specifically, the first vaccine given in New York in November 1978, and doctors in Manhattan first began to recognize cases of "gay"cancer” in young homosexual men in Manhattan in 1979 (with the first appearing in January 1979). The follow up trials (in Los Angeles and San Francisco) went from March 1980 to October 1981, while the first case of AIDS appeared outside of New York appeared in San Francisco in the October 1980.
Most importantly, a later 1986 study (which excluded every hepatitis B participant who subsequently developed AIDS) found that in the 1978 and 1979 blood samples of the trial participants, 6.6% had formed antibodies to HIV—indicating they had been exposed to HIV prior to the original AIDS case (that mysteriously appeared out of nowhere), and had the excluded participants (who developed active rather than silent AIDS infections) been tested, the 6.6% would have been much higher. Furthermore, that study showed in 1982 20% of them had HIV antibodies, and in 1984 40% did (while conversely, only 1 of the 87 with HIV antibodies subsequently developed AIDS) further supporting the argument those without AIDS from the vaccine trials still had been infected by the trials but simply had a much slower progression of the virus.
This sudden emergence of a disease killing young healthy men, in turn, immediately led to many doctors and patients being suspicious it was due to the Hep B vaccine, resulting in both the CDC and the vaccine manufacturer issuing numerous statements and reports in the ensuing years claiming the vaccine would not cause AIDS.
Additionally, it was noteworthy that the architect of the HIV trials (who had a very questionable background), out of the available cohorts with a high risk for HIV, specifically chose young healthy gay men in order to avoid "serious legal and logistical problems," and that the precise demographic he required for the study matched those in whom HIV originally emerged. Likewise, a colleague who knew gay men in those trials was told by the participants that they noticed throughout the trials there was a heavy degree of scrutiny on them (as though the researchers expected or were worried something bad would happen to them), and that they suspected homosexual men were chosen for the trial as they were less likely to have family members who would complain if they passed unexpectedly.
Note: I was able to verify many but not all of the claims put forth in the primary book (written by a dermatologist within the gay community) which advanced the above hypothesis.
For additional context, gay friends of a colleague in the original hepatitis B trials in those cities shared with him that they noticed during the trials there was a heavy degree of scrutiny on them (as though the researchers expected or were worried something bad would happen to them), and that they suspected homosexual men were chosen for the trial as they were less likely to have family members who would complain if they passed unexpectedly.
As best as I can tell, this contamination issue prompted Merck to eventually resolve the problem by creating the first recombinant (genetically engineered) vaccine, which involved growing the antigen in modified baker’s yeast. This vaccine entered the market in 1986. While I cannot prove this, I believe that chronology accounts for both why the public health leadership was so invested in the newer hepatitis B vaccine (as it solved a massive problem they had created, and like the subsequent mRNA vaccine, also represented a new groundbreaking technology that would advance the entire field of vaccines).
Likewise, I believe this explains why the hepatitis B vaccine initially was so expensive (as plasma-produced vaccine was costly, setting a high marker price, and initially GMO technology was relatively new and hence more costly)—although now in parts of the world with less financial resources, the hepatitis B vaccine is still produced from human plasma to save costs.
Note: it has also been hypothesized (and in the mainstream “debunked”) that the spread of AIDS followed oral polio vaccination campaigns (due to the vaccine being grown on contaminated monkey kidneys). Likewise, in 1990, the Times of London published an article (in consultation with the WHO and a military doctor who later became Trump’s CDC director) which noted that the spread of AIDS across the world mirrored the WHO’s smallpox vaccine campaigns—which led to them concluding that it triggered an immune suppression which caused a latent HIV infection to transform into AIDS (with immune suppression being an issue commonly seen with many vaccines).
The History of the Hepatitis B Vaccine
Note: much of what follows is from a thesis which, while having a heavy DEI focus, provides by far the most detailed (and well referenced) summary of events I have come across as to what happened with the hepatitis B vaccine.
Hepatitis has long been a challenge for medicine (e.g., as I detailed here, hepatitis virus-contaminated hot vaccine lots were a recurring issue with vaccination), to the point that the researcher (Blumberg) who discovered the hepatitis B surface antigen in 1965 won a Nobel Prize. Once discovered, blood tests for it were rapidly developed along with Blumberg, in the early 1970s proposing the original plasma-derived (and then heat-inactivated) vaccine and then developing it between 1971-1975.
Note: beyond there being a need for it (e.g., due to increasing rates of hepatitis B and an ever increasing ability to detect it due to the new test), hepatitis B was an ideal disease to make a vaccine against as beyond it being a serious concern to the medical profession, it had limited transmission and did not rapidly evolve to vaccine resistant variants (making it in theory possible to eliminate with mass vaccination).
From the start, the vaccine was intended to target high-risk groups, and as hepatitis B was a significant issue within the gay community, and by the mid 1970s, vaccine development had significantly accelerated thanks to the assistance of the gay community (e.g., they often donated plasma and participated in both efficacy trials and the first large trial—which was conducted by the New York Blood Center).
The successful NYBC trial was completed and then published in 1980, immediately receiving widespread praise from key figures in America. The next year, Merck released Heptavax-B (which was heavily marketed to the gay community—a move many felt was highly stigmatizing due to its linking promiscuity with death—but nonetheless the gay community engaged in widespread activism to increase vaccine availability and uptake). The following year (1982) ACIP then recommended it to high risk groups (e.g., gay or bisexual men, IV drug users and health care workers). However, vaccine use remains low (as the vaccine was priced at 145 for three doses (whereas other vaccines cost around 2 dollars) making it fairly unaffordable, particularly since many insurances and state programs would not cover it and at the time, it was not politically viable to implement widespread coverage for gay men (requiring them to pay for the vaccine with their own funds)—providing an incentive to add it to the childhood immunization schedule (as widespread funding for those vaccines already existed).
Note: in 1985, the vaccine was also recommended for heterosexuals with multiple sex partners or a recent STD.
In 1984, ACIP then recommended hepatitis screening in high-risk pregnant mothers so that their infants could receive the vaccine and immunoglobulins preventing vertical (maternal-to-infant) transmission. Following this (in the mid-1980s), extensive testing revealed higher than expected hepatitis B levels (which during the 1980s had increased by an alarming 37%), after which, ACIP, in 1988 recommended screening all pregnant mothers (as data showed only screening “at risk” mothers missed 35-65% of those with hepatitis B).
Note: ACIP attributed this increase to 85% of the vaccine going to only 3 “at risk” groups, such as health care workers (rather than those with the greatest need). However I suspect some of this increase was an artifact of increased testing rather than an actual increase given that chronic hepatitis B rates never rose.
Although the ACIP had previously favored increased outreach to high-risk adults, Margolis [chief of the CDC’s hepatitis division] argued for a new focus on the immunization of young people because they could “be accessed by prevention programs before high-risk lifestyles are initiated.” Even though adolescents were closer to the typical age of exposure to hepatitis B, parents were more likely to take their infants for regular doctor’s appointments, which made infant immunization predictable. Margolis recalled that the high rate of chronic infection among infants was also an important factor in the ACIP’s decision to shift to an infant-based strategy. The ACIP agreed with Margolis that infant vaccination was the best way to control hepatitis B, even though it might take 15 to 20 years to see the public health effects.
In 1990, ACIP then recommended vaccinating all children of high-risk mothers (e.g., refugees from areas with high rates of hepatitis). Then in 1991, citing the need for predictable access to infants through routine doctor visits and high chronic hepatitis B infection rates in early childhood, recommended the hepatitis B vaccine to all newborns along with advising all high-risk adolescents vaccinate (with the disclaimer that “vaccinating persons engaged in high-risk behaviors, lifestyles, or occupations before they become infected generally has not been feasible”). Finally, in 1995 the ACIP expanded the recommendation to include routine vaccination of all adolescents aged 11–12, and in 1999 to all unvaccinated individuals aged 0–18.
The thesis, in turn, provides references that the following factors played key roles in the rationale to give the vaccine to children universally:
•As shown above, ACIP had noted adult vaccination was “generally not feasible” due to cost and logistical barriers.
•The CDC concluded (based on epidemiological research showing a third of people with hepatitis B denied having any risk factors for it) that people were simply not reporting their risky behaviors—hence making targeted risk-based approaches not viable.
•In the early 1990s, adolescents (11 to 21 years of age) and young adults (22 to 39 years of age) had the highest rates of hepatitis B, something published articles attributed to them being the most likely to engage in unprotected sex and needle sharing.
Note: many references at the time showed this was the most significant issue within the gay, bisexual, and African American communities (particularly where those overlapped)—even once widespread fear of HIV had swept across America, likely leading the CDC to conclude it was beyond their capacity to perform targeted outreach to these large demographics.
These actions, in turn, were quite controversial as:
•Members of the public felt a vaccination campaign should not be extended to them for issues within a community they were not part of (e.g., when New Jersey’s legislature was debating an ultimately unsuccessful bill to require it for school entry, one State Senator stated, “They are trying to treat the wrong people with this vaccine…They can't reach the adults through education, so this is their solution. I think it is a poor one”).
•Many pediatricians were reluctant to accept ACIP’s recommendations that all US infants be vaccinated for hepatitis B (due to the numerous reasons it didn’t make sense), in turn, requiring roughly five years of comprehensive efforts to “educate” providers and parents of the necessity of the vaccine—at which point American pediatricians fully embraced it.
•Members of the gay community felt that better targeted outreach could have solved the issue (e.g., by working with the existing network of gay clinics and community health centers—something the CDC never did despite existing funding and previous research partnerships).
Or to quote the physician who testified in 1999 before the Ohio legislature about mandating the vaccine:
The rationale presented for universal vaccination of infants in the U.S. stemmed from the failure of the current strategies for controlling this disease and not from trials that demonstrated the effectiveness or safety of a universal hepatitis B vaccination program.
Why is the Vaccine Given?
Since the official reason for mass infant vaccination (preventing maternal-to-fetal transmission) is on extremely shaky ground, over the years, I have heard a variety of theories to explain this policy. The ones I have heard presented with the greatest certainty (and sometimes shared by “insiders”) include:
•Since the target demographics for the hepatitis B vaccine are a relatively small segment of the population, universal vaccination was needed to increase sales to a profitable and sustainable level.
•Vietnam has the highest rates of hepatitis B in the world (10% in urban areas and up to 40% in rural areas). After the Vietnam war ended in 1975, a large flood of Vietnamese refugees entered the USA, roughly doubling in both the 1980s and 1990s (to 988,000 immigrants by 2000), resulting in a sudden explosion of neonatal hepatitis B transmission within the United States once they had children here which authorities felt could not be addressed restricting neonatal vaccination to the high risk groups.
•While the National Childhood Vaccine Injury Act provides legal immunity to manufacturers who injure people with their products, it only does so for vaccines that are on the CDC schedule for children and adolescents. As such, due to the elevated injury rate of the vaccine, it was not financially viable to sell it unless it was “advised” for all American children and exempted from liability.
•By having parents vaccinate their newborn in the hospital, it conditions them to come in for their 2-month vaccination appointment and hence be compliant patients who routinely acquire medical goods and services.
•Since injuries from newborn vaccines will occur before parents have a clear sense of what is “normal” for their child, it makes it much harder for them to recognize the subtle harms that frequently occur following vaccination, again making it easier to increase patient compliance.
As a good, but ultimately uncertain case could be made for each of those, I was always undecided on whether any were the primary reason, or alternately, an “added benefit” which helped garnish the support needed to implement the program.
Recently however, a close colleague of one of the 1991 ACIP members who voted for all newborns in America to receive the vaccine contacted me to share that the ACIP member in 1991:
[privately] explained to me in confidence that the vaccine was added not to prevent vertical [mother to child] transmission but to get the vaccine administered to a “captive audience” before they could leave the hospital. Their fear was that it would be the only opportunity to prevent Hep B infection later in life to the highest-risk inner city youth populations.
Note: as this was shared in confidence with me (presumably in part because their ACIP colleague had wanted things to remain in confidence—which I suspect was due to the public pushback they knew their decision would elicit), I altered the statement I received to preserve the reader’s anonymity. In the original statement, they provided additional detailed information that corroborated their claims.
I, in turn, am inclined now to believe the CDC’s inability to manage hepatitis in at-risk communities was their ultimate rationale for vaccinating every newborn, while the “benefit to mothers” was an excuse created to justify it—particularly when you consider all the additional context presented throughout this article and the fact being hepatitis B negative is not considered a valid reason to skip newborn vaccination.
Conclusion
In my view, the saga of the hepatitis B vaccine exemplifies how the government often takes a top-down approach to addressing a challenging issue, even when this approach is not suitable for the circumstances. In turn, the pressure the CDC was under to do something about hepatitis B (given that each successive attempt had failed) made them conclude they had no option but to universally mandate the vaccine, and then to overcome public resistance to this, insist the vaccine was miraculous and completely safe. Because of this:
•Much of the foundational information needed to justify the policy was never researched, or if known made widely available to the public (in turn, illustrating why Senator Cassidy has strong beliefs in favor of the vaccine which are entirely at odds with the actual data on it)—something I believe accounts for why despite decades of newborn vaccination—the ultimate target of the program (chronic hepatitis B infections) remains unchanged.
Note: somewhat similarly, I spent a few weeks trying to figure out how deadly influenza infections were (to compare them to COVID infections) and eventually discovered to my great surprise that no one actually knows—despite the risk of dying from flu being trumpeted throughout the media each year to market the annual flu shot.
•All concerns about the safety of the vaccine were swept under the rug (or prefaced with statements such as there being “insufficient” evidence of harm), with all of the research that should have been done to assess this never being conducted.
•It is very likely that a safer hepatitis B vaccine (which does not trigger autoimmunity to human myelin) could have been developed. However, since a liability-free monopoly was created, there was never an incentive to do this.
•Parents are often forced or tricked into vaccinating at the hospital, greatly destroying their trust in the medical system and making more and more people wanting to skip hospital birth entirely. These for instance were five recent replies I received on Twitter when I asked about it:
Fortunately, an opportunity has at last arrived to correct this decades long catastrophe, as tomorrow, the ACIP (which for the first time is composed of independent members) will at last critically re-evaluate the vaccine and assess if every newborn should receive it if their mother does not have hepatitis B (which remarkably the entire medical profession is treating like an apocalypse). This is a once-in-a-lifetime opportunity many have dedicated their lives to correcting, and it is for that reason I spent the last two weeks drafting this article so the public could better understand what is actually up for debate.
As such, it is my sincere hope that you can help bring attention to the matter before tomorrow’s hearing (e.g., by contacting your Senator to express support for changing the hepatitis B recommendations or attending the ACIP Zoom meeting). Likewise, I thank each of you for the support that makes this type of work possible.
To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here.




Numerous readers in the comments here (and on this twitter post: https://x.com/MidwesternDoc/status/1968444962145784089) shared that either they or their child was significantly injured by this vaccine, making it impossible for me to pin all of them.
This underscores both how common these issues are, and that they likely far outweigh the 1 in a million hepatitis cases prevented by the vaccine.
Comment I received via email:
Hello,
I've wondered for years why Hep B vaccine has been given to newborns.
During the last four years of learning more about the deep state, I feel
they want newborns vaccinated period as it blocks them from remembering
who they truly are and causes their frequency to lower considerably.
Children born to humans on Earth during the last decade have been coming
in at a much higher frequency than those before them. They come in with
so much light, the deep state is afraid these children will be their
downfall. The DS controls big pharma. Both will lose. That's fine is
over.
Much Love to you for all the hard work you do bringing the truth to us
all.