What Do We Now Know About Hot COVID-19 Vaccine Lots?
Every once in a while you see a meme that requires an article to be written on it. I recently came across this one while working on an article attempting to explain why vaccines cause sudden death.
One could thus argue Neo’s ability to dodge in this cartoon illustrates:
•Having walked outside the matrix of believing vaccines are perfectly safe and effective.
•All COVID-19 vaccines being potentially deadly and the refusal to comply with one of the most aggressive propaganda campaigns in history or any vaccine mandate regardless of the consequences for doing so. In many cases resisting being vaccinated was analogous to escaping from an army of Agent Smiths.
•Specific lots being deadly and one being able to avoid taking the bad batches.
•Vaccine susceptibility being dependent upon pre-existing health status of the recipient (in other words, a healthy internal terrain makes you more likely to dodge the most severe side effects).
In this article I will focus on the lots themselves; the other points will be addressed in the upcoming article.
My Experiences with the COVID-19 Rollout
When the COVID-19 vaccines entered the U.S. market, I expected them to have a much poorer risk/benefit ratio than had been marketed. In most cases, new vaccines fail to meet the promises that are made in order to gain support for an immunization campaign, and in the case of the COVID-19 vaccines, given the many unique challenges of the time, it was completely unrealistic to expect the vaccines could be sufficiently developed before they were rushed to the market to “end” the pandemic.
I also felt it was likely that most of the serious issues from the vaccines would show up far after the time of vaccination, as there were serious concerns their design could cause numerous autoimmune diseases, issues with fertility or pregnancy, cancers and would likely lead to the population becoming unable to develop herd immunity to COVID-19, thereby making vaccinated individuals become more susceptible rather than less susceptible to COVID-19 as time moved forward (which has now happened). There was a strong scientific basis for these concerns and many had sounded warnings on each of them that were not heeded by the regulatory agencies.
In December of 2020, documents from the EMA (Europe’s FDA) on Pfizer’s vaccine were leaked. Because I had previously worked in drug development, I eagerly read through them. Although I recognized that there was a certain degree of urgency to expedite the approval process, I was nonetheless astonished that Pfizer was allowed to omit many key animal tests that are required prior to conducting human vaccination trials—the teams I worked with to develop therapeutics for COVID-19 were held to a much harsher regulatory standard.
I particularly took notice of the fact that tests to evaluate key issues that had already been identified with the mRNA vaccines (cancer, autoimmunity, infertility, ineffective protection against variants) had not been conducted. The European regulators who were also aware simply “invited” but did not require Pfizer to conduct further studies to address those concerns. I interpreted this as a tacit admission the vaccines would have major problems in these areas and Pfizer felt their best option was to simply have no available data (a common reason why dangerous drugs make it to market is because pharmaceutical companies in most cases will not publish datasets that show their drug is harmful, and every now and then we discover this through lawsuits).
Once the vaccines entered the market, I began to have friends from around the country contact me to inquire if the vaccine could cause fatal heart attacks or blood clots because this had happened to one of their loved ones or close friends. After the magnitude of the problem dawned on me, I tried to warn my colleagues in medicine and discovered that very few were receptive to anything questioning their faith in this modern day scientific “miracle.”
Not knowing what else to do, I began documenting each case that came my way (which included approximately 50 deaths and countless more severe injuries) so I could have some type of proof to present to my colleagues that the injuries people were reporting online were real and to have a way to honor the experiences of my friends and associates who had been severely injured by these vaccines.
I later posted my documentation here, by a stroke of luck it ended up being seen by many, and it ultimately became one of the countless safety signals that should have been listened to but was ignored (the total number of injuries contained solely within my own log in the past had been sufficient for regulators to consider pulling a vaccine from the market).
Although I am biased towards being skeptical of a pharmaceutical’s safety, the immediate wave of severe adverse reactions from the vaccines was completely outside my expectations. I was quite alarmed because these numbers indicated the vaccine trials were much more deficient than I had initially expected (deaths and anaphylaxis are easy to catch, so if they were not recognized, then most of the easier to miss side effects were likely not recognized either). It must also be remembered that typically with a toxin that affects numerous systems in the body, acute fatalities tend to be a relatively rare side effect compared to the chronic complications that also emerge. This unexpected excess of deaths following vaccination thus suggested the chronic complications were going to be much worse than I had initially expected.
Previous Toxic Vaccine Lots
As I began logging these events, I noticed in certain parts of the Midwest that I would hear of reports of two immediate family members (e.g. a husband and wife) both having severe or fatal vaccine injuries. It seemed highly, highly, improbable this could happen by chance, and instead argued both had been vaccinated at the same place and both had received a more toxic vaccine lot.
At the same time, I and colleagues began noticing sharp demarcations in the reactions to the vaccines; some individuals seemed as though nothing had been injected into them, while many others had a severe inflammatory response that was completely different from what they had experienced with any previous vaccine. These observations argued for some of the vaccines being placebos.
This opened a train of thought into the potential parallels between this vaccine and the two previous vaccines that were suspected to have had hot lots. We will now review what happened with the Diphtheria-Pertussis-Tetanus (DPT) vaccine and the Anthrax Vaccine.
DPT and Anthrax
DPT is a remarkably dangerous vaccine which has been repeatedly associated with death following vaccination (in this article which contains many references for this section, I presented the century of evidence substantiating that link). DPT has also been linked a variety of severe neurological disorders, and those injuries have affected both my own family and the families of many of the readers here.
One of the interesting historical events with the DPT vaccine occurred in 1978—79, where eleven babies in Tennessee were found to have died within eight days of a DPT vaccination. Nine of the eleven had been vaccinated with the same DPT lot, Wyeth’s #64201, and five (four from the same lot) had died within twenty-four hours of vaccination. Two important events occurred as a result of public recognition of this hot DPT lot.
First, in June, CDC director Foege wrote a memo to the Surgeon General stating that the experts “did not feel that a causal relationship had been established between vaccination with DPT from Wyeth's lot #64201 and sudden infant death in infancy. However they did not feel that a causal relationship could be totally excluded.”
Secondly, Wyeth appeared to have attempted to address this problem by acting to prevent a clustering of deaths following DPT vaccination from a single lot from ever occurring again.
On August 27, 1979, a Wyeth official wrote in an internal Wyeth memo, “After the reporting of the SIDS cases in Tennessee, we discussed the merits of limiting distribution of a large number of vials from a single lot to a single state, county or city health department and obtained agreement from the senior management staff to proceed with such a plan.”-
The memo revealed that Wyeth would attempt to distribute no more than 2,000 packages of vaccine from one lot number to a single destination. Another 1983 memo confirmed that policy of limiting shipments of DPT vaccine from a single lot to a geographical location, and also referred to the “SIDS episode.”
The vaccine safety movement has long believed that this incident marked the point in time where the government was clearly aware vaccines could cause deaths but would choose to cover them up instead of admitting them. More importantly, it also marked the point at which the vaccine industry realized “the solution to pollution is dilution.”
The industry was henceforth able to mask future incidents like the Tennessee hot lots from coming to public recognition by spreading their deadly lots throughout the country. By doing so, it became very rare for individuals who witnessed a vaccine death to also witness other deaths occurring within their community and thus potentially connect the deaths to vaccination. Until the recent COVID-19 campaign, this strategy appears to have been remarkably effective.
The Anthrax Vaccine
Following the Gulf War, over one hundred thousand veterans became extremely ill with a variety of severe ailments that often resulted in permanent disability. A variety of factors were proposed to explain what caused Gulf War Syndrome, and of these I am certain the anthrax vaccinations were the primary culprit (as it is the only variable that can account for everyone who was affected and unaffected, including many who came down with the same disease long after the Gulf War had ended). Today, a sitting member of Congress suggested the same:
I strongly believe the anthrax vaccination campaign in the 1990s (which was given on an “emergency” basis to the military) was the prototype for the COVID-19 vaccination campaign. The anthrax program was orchestrated by corrupt elements within the military and H.H.S. (many of whom remained in power and were key figures with the COVID-19 vaccine campaign), hundreds of thousands of servicemen were vaccinated against their consent, over a hundred thousand were severely injured, and despite diligent efforts by veterans and congress, the military continues to gaslight these victims and insist the anthrax vaccines were not the cause of their injuries.
It is thus quite valuable to study what occurred back then as it serves as a case study that can teach us much about what happened now. Additionally, had the architects of anthrax vaccination campaign been held accountable for what they did, it is almost certain that much of what transpired during the COVID-19 vaccine campaign would never have been permitted. This underscores the importance of holding those responsible for COVID-19 and the spike protein vaccines accountable for their actions so something like this cannot once again happen in the future.
Although I am relatively certain the anthrax vaccines themselves were the cause of Gulf War Syndrome, I am less certain why they were so problematic. Having looked at this topic extensively, I believe there are three hypotheses to explain what happened.
Squalene Hypotheses
The first, as told within Vaccine A and covered in more detail within this previous article, is that the federal government needed to develop new adjuvants for many experimental vaccines that Fauci was investing significant NIH resources into developing (e.g. one for HIV), as many of these new vaccines were chemically incompatible with the existing aluminum based adjuvants. Oil-based adjuvants were compatible with this new generation of vaccines but had a challenging toxicity profile that had significantly slowed the adjuvants’ development.
When the Gulf War started, the military needed an anthrax vaccine to guard against the risk of Saddam Hussein using anthrax on our soldiers; however, according to Vaccine A, the only feasible vaccine candidate they had to meet this need (for both efficacy and the ability to produce a large number of doses in the short time available) was an experimental anthrax vaccine that used squalene (an oil) as an adjuvant.
When those vaccines were administered to the servicemen, many of them noticed the procedure was suspicious. The servicemen were either not informed what vaccine they were receiving, or they received very vague documentation regarding its content (it was simply referred to as Vaccine A on their vaccine card), and in most cases the vaccines did not show up in their medical records.
A physician outside the military who had numerous patients with Gulf War Syndrome realized that they were all showing signs of complex autoimmune diseases she eventually traced to “Vaccine A.” With further research, she realized the symptoms they were experiencing were consistent with what could be expected from injection by an experimental squalene adjuvant and later still a different doctor who developed almost identical symptoms after participating in a much earlier vaccine trial that utilized a squalene adjuvant also came forward .
Following her realization there may be a major issue with squalene in the vaccines, the physician located a colleague who was able to develop a test for antibodies to squalene (something expected to follow injection of squalene). This test demonstrated that the servicemen affected with Gulf War Syndrome (and those later who developed the same disease after receiving an anthrax vaccine long after the war had ended) had antibodies to squalene that did not appear in those who were unaffected by the disease. Later still, they were able to demonstrate these antibodies did not exist prior to injection of the anthrax vaccine but did afterwards (as servicemen who were being forced to vaccinate by the military volunteered to serve as subjects to test this hypothesis). Given this, unless a component of the anthrax bacteria has a homology to squalene that independently produced the antibodies (which is possible but fairly unlikely), the results of that test prove squalene was present in the anthrax vaccines.
Despite mounting concerns against the anthrax vaccine following the Gulf War, it continued to be used on an experimental basis, with the most notable instances being in the late 1990s at military bases around the country, and in the second Iraq war. Dover Air Force Base in Delaware (Biden was Delaware’s senator) was chosen as one of the initial sites to receive the vaccine and many complex vaccine injuries that resembled Gulf War Syndrome immediately followed its roll-out there.
The commander of the base Colonel Grieder requested answers from the Pentagon and after being rebuffed, suspended the program. Six days later, senior officers of the military held a town hall at the base where they cited the safety and efficacy of the vaccine and unequivocally denied the presence of squalene within it (video footage exists of this meeting). Oddly however, one of the senior officers did briefly admit the military possessed an experimental anthrax vaccine with squalene but stated that it had not been used on anyone at Dover. Taking them at their word, Grieder reinstated the program
The FDA subsequently tested some of the suspect vaccine lots for squalene and in September of 2000, revealed it was present in 5 lots (4 of which had been sent to Dover). Following the FDA testing, the military tacitly admitted the presence of squalene but insisted on its safety. Later still, squalene became an adjuvant used within commercially available influenza vaccinations (including some associated with severe adverse events), and later still could be found within some of the candidate COVID-19 vaccines funded by Operation Warp Speed (this was where the concern that COVID-19 vaccines would kill many sharks came from as sharks are a major source of squalene).
In addition to the FDA finding squalene in the vaccines, independent squalene antibody testing in vaccine recipients also accurately predicted its presence in those lots, and identified three other suspect lots (two of these had been sent to Dover) which were never formally tested for squalene. Later, during the mobilization for the second Iraq war, a large number of anthrax vials were dumped overboard, likely by protesting soldiers, and when a news program had them tested by an independent lab, squalene once again was found to be present.
One of the most concerning aspects of the FDA’s test results lies in the exact data that was obtained.
Lot FAV 020 had 11 parts per billion of squalene
Lot FAV 030 had 10 parts per billion of squalene
Lot FAV038 had 27 parts per billion of squalene
Lot FAV043 had 40 parts per billion of squalene
Lot FAV047 had 83 parts per billion of squalene
When an experimental drug is being tested out on humans, the first step is to perform phase 1 trials, which test the safety, side effects, best dose, and timing of the new treatment. Dose response studies using a dilution series (i.e. 1:2:4:8) are frequently performed for this purpose and typically coded by researchers in a logical code that is easy to track. With these lots, it is quite noteworthy that the squalene concentrations also followed a dilution series that ascended in order with the lot number. It is extremely improbable this pattern could have emerged by chance.
Alternative Anthrax Vaccine Hypotheses
Meryl Nass MD is arguably the physician with the most expertise in the world on the anthrax vaccination and previously worked with congress when the program was being investigated (it should be noted the military was uncharacteristically resistant to any type of congressional oversight of this vaccine program).
Although I found the evidence for the squalene hypothesis compelling, Nass’s opinion was that it may have been a product of confirmation bias, as the concentrations of squalene found in the vaccines were low to the point it is difficult to be certain it could create a physiologic response (modern squalene based vaccines use a much higher adjuvant concentration).
Nass’s hypothesis was that the Anthrax vaccine’s toxicity arose from it being an inherently dirty vaccine because its manufacturing process (which involved culturing, killing, and then purifying large numbers of anthrax bacteria) produced a substance that was highly likely to produce adverse reactions in the recipients and was difficult to filter during the purification stage because it would frequently clog the vaccine filters.
The vaccine manufacturer, Bioport, chose to address the filtration issue by using larger filters which did not become clogged by the vaccine product. This choice led to the filters failing to fulfill their function and the final vaccine product containing a variety of toxic components that should not have made it that far. It should be noted that Nass was able to review documents directly verifying the previously mentioned events at Bioport occurred. The FDA also had concerns over Bioport’s quality control and repeatedly cited Bioport for their manufacturing processes and suspended shipments of the vaccine from their facility.
The final hypothesis I have come across (from Garth Nicholson) was that the anthrax vaccine’s pathology was a result of a weaponized mycoplasma he was aware of prior to the Gulf War but also later detected in the Gulf War veterans. This mycoplasma was either present in the vaccine (as far as I know no anthrax vaccines were ever tested for its presence) or became active following a period of immune suppression. Receiving multiple vaccines concurrently is immunosuppressive, and many soldiers had multiple immunizations immediately prior to being deployed for the Gulf War. For those interested, this video summarizes many of Nicholson’s findings.
Although this is probably the most speculative theory of the three, I believe it deserves mentioning because I have spoken with multiple Gulf War veterans who told me Nicholson was the only person who was able to help them (the antibiotic Nicholson had previously identified that addressed this mycoplasma species significantly improved their symptoms), and in many cases these veterans observed Gulf War Syndrome spread into the family of the veteran, something which can only be explained by an infectious agent.
Interestingly, the same antibiotics Nicholson used to treat the mycoplasma infection (doxycycline and azithromycin) were found to treat COVID-19 and Nicholson has produced data which argued some cases of COVID-19 were worsened by a concurrent infections with these mycoplasma. Similarly, these antibiotics are also used to treat Lyme disease, and the symptoms of the mycoplasma disorder appear to overlap with a variety of chronic complex conditions including Lyme disease and certain COVID-19 vaccine injuries.
Based on my current understanding of vaccine pathology and the clinical signs observed in veterans with Gulf War Syndrome (particularly Andrew Moulden’s observations which will be discussed in the next article), I believe Nass’s hypothesis is the most probable, but I simultaneously find the other two to be extremely compelling.
Lessons From the Anthrax Vaccination
Let us now consider how the stories of these vaccines are relevant to COVID-19.
From DPT, there is a precedent for a hot vaccine lot to not be removed from the market and instead for its toxicity to be concealed by disseminating the lot throughout the country. As a result, the only way a dangerous lot could be detected by the general public would be if the VAERS data for the entire country (sorted by lot number) was examined, or an outside observer like myself noticing a pattern of family members who lived together (including unrelated ones such as a husband and wife) developing severe vaccine injuries.
From anthrax, we have shown that if there is a dire commercial need to develop a new but potentially unsafe vaccine technology that was funded by the government, there is a precedent for an emergency situation to be utilized by criminal elements within the government to test and develop that technology. Those tests then can be expected to employ varying doses of the novel technology to establish what concentration is high enough to get the required effect but low enough to avoid an intolerable toxicity. Ideally, these doses would also have a code or order that could be used by the researchers to identify them.
In addition to the mRNA technology perfectly fitting this description (there is a profound commercial need within the pharmaceutical industry to bring mRNA to market, but despite decades of research this was not possible due to the high toxicity of the mRNA product), the same could also be said for the lipid nanoparticles needed to bring mRNA into cells.
I also suspect that the anthrax campaign and the concurrent commercial push to develop the oil-based adjuvants (oils are lipids) played a key role in paving the way for the lipid-based nanoparticles needed to deliver mRNA inside cells (these particles also function as adjuvants for the vaccines). It should be noted that throughout the leaked regulatory documents I reviewed, the toxicity of the lipid nanoparticles was never independently evaluated and the only consideration the regulators gave to this issue was mentioning a concern that one component, their acetamide moiety, was known to cause liver cancer.
Contaminated Lots
One of the major issues within the vaccine debate is how little is understood about how vaccines are made and that their production process makes it quite challenging to ever produce a “clean” vaccine. I believe many of the issues with vaccinations arise from their production being an inherently dirty process which will inevitably produce contaminated vaccines, that lot by lot, will vary in their degree of contamination.
In addition to Nass’s discovery with the anthrax vaccines, there are many other historical examples of serious vaccine production issues. For example, the polio vaccines were grown in kidney cells from monkeys and many were later found to be contaminated with the SV-40 virus, a virus known to cause cancer. In the case of the with the DPT vaccine:
The FDA’s pertussis vaccine specialist, Charles Manclark, commented in 1976: "Pertussis vaccine is one of the more troublesome products to produce and assay. As an example of this, pertussis vaccine has one of the highest failure rates of all products submitted to the Bureau of Biologies for testing and release. Approximately 15-20 percent of all lots which pass the manufacturer’s tests fail to pass the Bureau’s tests.”
One of the best summaries I have found of the contamination issues inherent to vaccines can be found in Chapter 7 of Fear of the Invisible:
“In 1998 and 1999 scientists representing the World Health Organization (WHO) met with the senior vaccine regulatory scientists from the USA and UK at the National Institutes of Health (NIH) in Washington D.C. to discuss the safety of the manufacturing methods employed to produce vaccines. No journalists were present, but official transcripts were kept. What they record is that all the many experts that spoke expressed grave concern over the safety of the manufacturing process currently employed to make the licensed vaccines, such as MMR, flu, yellow fever, and polio. It was reported by leading experts that the vaccines could not be purified, were “primitive,” made on “crude materials,” and the manufacturers could not meet lowered government standards. WHO specialists reported the widespread and continuing presence in the MMR vaccine of chicken leucosis virus. Others spoke about the presence of foamy virus, many other viruses, toxins, foreign proteins, enzymes and possibly prions and oncogenes, (which, being of equal or smaller size than the desired viral vaccines, cannot be filtered out). Grave concerns were expressed about the levels of foreign residual DNA and RNA contaminating the vaccines. It was feared that this (contamination) could be causing cancers and autoimmune diseases.”
In both industry and government, I have found they frequently encounter situations where a problem exists that requires a solution, but the tool available to address the problem has serious shortcomings. In those situations, rather than refine the tool or utilize a different approach, the typical response will be to use the tool and employ the power of industry or the government to push through its shortcoming (this is very similar to using a hammer to pound a square peg into a circular hole rather than making your peg match the hole).
The division of the FDA that is in charge of inspecting the production of injectable biologics (which include vaccines and monoclonal antibodies) is woefully understaffed and cannot perform most of the inspections they are required to perform. More importantly, as whistleblowers have attested, when serious quality control failures are found that put patients at risks, the FDA will rarely if ever act on the findings of its investigators and request the manufacturer to improve their grossly negligent practices that jeopardize the safety of patients being injected with these products.
Consider the case of the plant that is the largest manufacturer of sterile injectable controlled substances in the United States. It has been repeatedly cited by the FDA over the last decade for bacterial and fungal contamination issues which in some cases has led to recalls, yet since the US drug supply depends on this plant, it has never been shut down. Pfizer has run this plant since 2015, failed to fix its issues, and it is one of the primary manufacturing sites for the COVID-19 vaccine.
Not surprisingly, when Operation Warp Speed was announced, many suspected there would be serious issues in monitoring the production quality of its numerous vaccines (most of which ultimately never made it to market). The timeline that was followed for developing these vaccines was completely unrealistic and guaranteed to produce vaccines with serious shortcomings there would not be time to address. A few subsequent events appeared to confirm these suspicions:
•In April 2021, the FDA was forced J&J to suspended production of their vaccine due to quality control issues at the manufacturing plant and forced J&J to dispose of 60 million doses that had already been produced. Interestingly, the company that was responsible for producing J&J’s vaccine, Emergent BioSolutions, was previously known as Bioport, the company responsible for the anthrax vaccine fiasco (which again underscores just how little accountability government vaccine contractors face).
•In September 2021, Japan suspended the administration of one Moderna vaccine lot after visible steel particles were found within them.
•On 11/2/21 the BMJ published an article corroborating a Texas whistleblower’s accusations of data fraud in Pfizer’s vaccine trials (many of which were subsequently reported later confirmed at a key Pfizer trial site in Argentina). It was revealed that she repeatedly tried to convince her superiors to address the data integrity breaches she observed (as they could incur significant penalties from the FDA for failing to do so) and after her superiors repeatedly ignored her warnings, she reported the issues directly to the FDA. The FDA chose to ignore the report, reported the whistleblower to her supervisors and never inspected her trial site. Ultimately, the FDA only inspected 9 of Pfizer’s 150 trial sites and 1 of Moderna’s 99 trial sites; which raises serious questions over what had to have happened to trigger the few (non-punitive) inspections that did happen and the production quality of the vaccines that made it market.
From this section, I hope to make the point that serious production issues can occur with vaccinations, and in most cases almost nothing will be done about it other than industry and government working together to cover them up.
mRNA Integrity
When the COVID-19 vaccines originally entered the market, they were held under lock and key in order to make sure they were always kept between -130°F to -76°F. Over time, this requirement became somewhat forgotten and temperature control of the vaccines is no longer as strictly followed. This section will explain why that occurred.
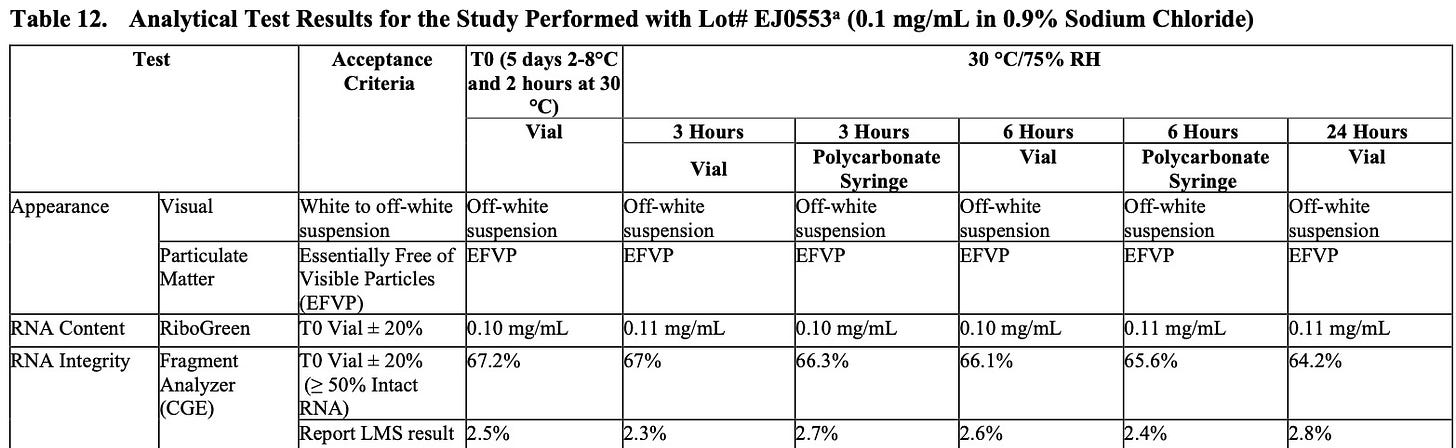
As mentioned before, the European drug regulators gave Pfizer a free pass on many major shortcomings of their drug application (hence why it took us so long to discover the vaccine concentrated in the ovaries). However, there was one area where the drug regulators repeatedly expressed major concerns: the mRNA’s integrity.
When I reviewed Pfizer’s internal data, it appeared that the mRNA integrity ranged from 51-71% percent depending on the vaccine lot (this included many lots that were subsequently injected into the human population). This means that much of the mRNA that was present was not the complete mRNA sequence that would produce the entire intended spike protein. These partial mRNA fragments (termed truncated mRNA), in turn appeared to have two potential consequences (these are based on my own understanding of the subject—the regulators only asked for but did not specify the potential consequences of truncated mRNA).
The first is that it would either not produce a protein or produce an incomplete spike protein, in both cases causing the vaccine to produce a lower than intended amount of complete spike protein (which is produced from the intact mRNA) and thus a lower than expected antibody response. The second was that the truncated mRNA species could produce potentially pathologic proteins and there was no practical way to assess the safety of these proteins (remember that the spike protein has multiple toxic components and since there are so many different ways the mRNA could be truncated it is impossible to test each of them). Alternately, truncated RNA could also be beneficial by making the vaccine less likely to produce deadly spike proteins (while I have no data here, a colleague who has to administered traditional vaccines to parents who requested them found they the vaccines significantly less harmful if they first became “expired” by sitting outside of the fridge).
The EMA documents were from the end of 2020 (long after human trials had begun) and at that point in time, Pfizer had not yet addressed the regulators’ key concerns regarding the truncated mRNA, and I suspect also did not do so in the short time that followed prior to the vaccines entering the market. Pfizer did however demonstrate that the integrity of the mRNA rapidly degraded once it was no longer kept in super low temperatures, which likely explains why there initially was such a strong push to maintain the correct storage temperature for the vaccines.
One of the key issues pharmaceutical companies have to overcome is to developing a method of production that allows them to rapidly produce the drug product that will be sold to the public (as the initial production method is almost always too slow) and produces a product without significant quality issues. Outside commentators thus argued (and in many cases with evidence) that the production quality and temperature control for the mRNA product was almost assuredly much better within the vaccine trials than in the vaccines provided to the general public that were produced under nearly impossible time constraints that required utilizing far less precise production methods.
Thus, when you consider all the potential areas that could cumulatively affect mRNA integrity within the civilian vaccine lots, it becomes a realistic proposition that truncated mRNA is playing a major role in the variability in response to vaccination being observed in the population. Unfortunately, while the regulators expressed grave concerns in this area, they ultimately appear to have chosen to do nothing about them, and instead as good regulators do, attempted to conceal the issue from the public.
For those wishing to know more about this topic, I would advise reading Steve Kirsch’s referenced article and the BMJ’s summary of these documents. Additionally, if you review the EMA documents directly, most of the key sections can be found by searching them for “truncated” and “mRNA integrity.”
Analysis of Individual Vaccines
One of the major challenges with analyzing the vaccines is that commercial labs are put in the challenging position that if they test a vaccine and publish the results, they can be penalized for doing so by the government and lose their ability to conduct their business. For this reason, whenever I or someone else looks into having a laboratory test a vaccine vial, even if there is interest in doing so, they typically decline to avoid exposing themselves to that risk.
Similarly, there are a variety of issues with having a laboratory legally obtain possession of a vaccine and test it (this was particularly challenging early on when the vaccines were kept under lock and key). Nonetheless, Steve Kirsch’s colleague recently was able to do so with mass spectrometry and made an interesting discovery. Of the 4 vaccines that were tested, (2 Moderna and 2 Pfizer), all appeared to contain the lipid nanoparticles, but none contained any mRNA. This suggests some lots are placebos and once again argues for significant lot-to-lot variability.
There have also many videos of vaccines being tested under microscopes that have shown a variety of concerning findings. Although some of the findings on these appear extremely concerning, I know at least some of the identified objects are things commonly found under microscopes that are not being recognized as common contaminants and hence am unsure what to make of these observations. However, while there is some room for misinterpretation with microscopy, to the best of my knowledge, the same cannot be said for the recent mass spectrometry results.
I have also heard many interviews from researchers who argued these are very dirty vaccines because they had analyzed the vaccines and identified a variety of contaminants. Although I suspect these reports are truthful, I do not have any evidence to verify the claims of each speaker and hence cannot present their claims as evidence here.
VAERS
Although VAERS clearly shows the presence of hot lots, to the best of my knowledge, data is not available on how many of each vaccine lot has entered the market. Hence the existence of a hot lot that has ten times as many fatalities associated with it could simply be the result of ten times the as many of that lot having entered the market.
Initially, I saw two potential ways to further evaluate this question:
•First, if the most likely comparison is drawn, and thus all deaths following an influenza vaccination over the last ten years are looked at, of the 274 deaths that occurred where a lot was known, 1 lot had 3 deaths associated with it, 14 lots had 2 deaths associated with it, and 243 lots each had 1 death associated with it, thereby showing there was no indication of a hot lot (which to some extent makes sense as deaths following influenza vaccines are rare and there are many different influenza vaccines on the market). This is very different from the pattern observed with the COVID-19 lots, where a few lots have had far more deaths than the rest.
•Second, there have been many anecdotal reports of individuals who had severe vaccine reactions looking up if they had a bad lot, and reporting their adverse event was the consequence of a bad lot. I am less confident in this piece of evidence because I have friends who were forced to vaccinate, made a point to get a lot that was a “safe lot” and then subsequently suffered a severe vaccine injury.
One analyst, Craig Paardekooper came up a very compelling analysis of the hot lots in VAERS that did not require knowing how many vaccines were present in each lot:
The major challenge with the mRNA technology that was well known prior to COVID-19 was finding an mRNA dose that was high enough to ensure a sufficient amount of the target protein was produced inside the body, but also low enough to ensure toxicity did not result (which was a very difficult balance to meet with mRNA and hence why it had not entered the market for decades).
In the above video, Craig made an excellent case that a dose response study had been conducted with these vaccines, which as mentioned above, also appears to have occurred during the anthrax vaccination campaign. More importantly, Craig’s data suggests all three manufacturers worked together to conduct these experiments. Subsequently Craig also made the case that, like the anthrax vaccines, Pfizer’s lot numbers followed a fairly simple code that correlated to dose of the experimental toxin within them.
When I discussed Craig’s finding with a mentor (I was quite disturbed by these videos), she asked me why I was so surprised by all of it and stated:
”The Federal Government has put an enormous amount of money into developing these vaccines. They are going to want a lot of data in return.”
As a final note, although many observers have pointed out that the FDA Pfizer documents show different mRNA doses are being used, these are simply a reflection of smaller doses being used in younger age groups (similarly, Moderna also used a higher mRNA dose than Pfizer). This document demonstrates that it is known different mRNA doses are needed depending on the recipient, but it does not prove the existence of hot lots.
Postscript:
Following publication of this article, a reader informed Pfizer was also extremely concerned about the potential toxicity of their lots and specified the lots would not be serialized (serializing makes it possible to trace reactions to specific lots and to know the lot’s size) in the contract governments around the world had to sign to get Pfizer’s vaccine. From a quality control standpoint, this is a major red flag, but as you have seen, quality control has not been a priority for these vaccines.
Thank you for taking the time to read and share this article. I’ve gone back and forth on if I want to join any social media platforms as the risk of having an account cancelled does not seem worth investing time into the platform, but since a few platforms do not appear to engage in that conduct, I am going to. If you would like to follow me on GETTR or Gab or reshare this article there, you are cordially invited to do so.



Key animal tests and biodistribution tests were not done as there is no agreed standard for them as yet for gene editing or gene replacement therapies - which mRNA injections are:
https://awkwardgit.substack.com/p/why-no-meaningful-biodistribution
On here is listed those organisations who agree to follow the guidelines on drug trails:
https://www.ich.org/page/members-observers
https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf
Bill and Melinda Gates Foundation are on the list.
There also a lot of in-depth documents on how to run trials:
https://www.ich.org/page/search-index-ich-guidelines
All of which are being ignored for covid vaccines.
Section 1.1 states:
"1.1 Adverse Drug Reaction (ADR) In the pre-approval clinical experience with a new medicinal product or its new usages, particularly as the therapeutic dose(s) may not be established: all noxious and unintended responses to a medicinal product related to any dose should be considered adverse drug reactions (my highlighting) . The phrase responses to a medicinal product means that a causal relationship between a medicinal product and an adverse event is at least a reasonable possibility, i.e., the relationship cannot be ruled out (my highlighting). Regarding marketed medicinal products: a response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of diseases or for modification of physiological function (see the ICH Guideline for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting).”
So in other words - with experimental medicines err on the side of caution and assume the problem was caused by the drug unless or until proven otherwise.
MHRA and other regulators are not doing this but the complete opposite.
Why?
Then there is this, the ICH have been trying to agree trials standards (since mid-2021) for gene therapies:
https://database.ich.org/sites/default/files/S12_Step2_Presentation_2021_0618_0.pdf
https://database.ich.org/sites/default/files/S12_FinalConceptPaper_2019_1118.pdf
Which is part of following on from this in 2018:
https://www.iprp.global/working-group/gene-therapy
And 2015:
https://admin.iprp.global/sites/default/files/2018-09/IPRF_Gene_Therapy_WG__Meeting_Summary_May_2015_0.pdf
So no agreed requirement on what biodistribution studies are required for gene therapies, which the Pfizer and other mRNA jabs and treatments are, so that is why in the EMA’s public assessment report it states “not required” or something similar.
https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
Notice also that the MHRA is not on the list of participants.
Why?
Because the EMA is and under CHMP if they say “all’s good, approve it” then the MHRA just says “OK will do” and rubberstamps it.
Admitted to in a FOI to me.
And just to give you all the EMA PARs used to issue the EUAs in the EU and UK here are the other 3:
https://ema.europa.eu/en/documents/assessment-report/spikevax-previously-covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf
https://ema.europa.eu/en/documents/assessment-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-public-assessment-report_en.pdf
https://ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf
I'm beginning to be very thankful I was born in a time when very few vaccines were given to kids. Also the time when this horrid corruption in agencies was far less entrenched and honour and honesty were still admired.